Chemical Composition Analysis
Chemical composition testing is a fundamental aspect of various industries, including manufacturing, materials science, metallurgy, and quality control. Understanding the precise elemental composition of materials is crucial for ensuring product performance, compliance with regulations, and maintaining high-quality standards. Here, we will delve into the chemical composition of steel, chemical composition metal, focusing on cutting-edge analytical methods such as X-ray Fluorescence (XRF), Optical Emission Spectroscopy (OES), carbon sulphur analysis, and nitrogen oxygen analysis. These advanced techniques provide invaluable insights into the elemental and molecular constituents of diverse materials, enabling industries to make informed decisions and achieve superior product outcomes.
An Overview of Steel: Definition, Composition, and Applications
Introduction to Steel Steel stands as a robust and adaptable metal, formed primarily from iron alloyed with a hint of carbon, and sometimes other elements. The inclusion of carbon lends steel its core strength and resilience. However, steel's versatility is further heightened by the introduction of elements such as chromium, nickel, molybdenum, and silicon, imparting attributes like abrasion and corrosion resistance. Indeed, steel's diverse range ensures there's a type fit for virtually every purpose.
Basic Composition of Steel At its core, steel is predominantly iron combined with a carbon content that doesn’t exceed 2%. Its wide range of alloys, however, is created by adding elements like chromium, manganese, and nickel to its base.
A Glimpse into Steel's Past The tale of steel finds its roots in the history of iron. The advent of the Iron Age, starting roughly around the 12th century BCE, marked the increased utilization of iron. Europe caught up by the 5th century BCE. Though China began producing early steel variants during this time, its iron content was too low for it to qualify as true steel. In India, around 400 BCE, genuine steel emerged from the combination of iron and charcoal.
Over the centuries, the development of steel and its richer cousin, cast iron, expanded globally. Noteworthy is the innovative method introduced by Benjamin Huntsman in England in 1751, which employed coal for heating. A significant evolution came from Henry Bessemer in 1855 with a new steel-manufacturing technique. This method became the foundation of modern commercial steel production.
The 20th century saw the birth of stainless steel, around 1912-1914, with the inclusion of chromium and nickel.
Key Constituents of Steel Steel's foundational components are iron and carbon. Other alloying ingredients lead to a myriad of steel grades. For instance, while mild steel primarily comprises iron, stainless steel might contain around 70% iron combined with significant amounts of chromium and nickel.
Production Processes for Steel Two principal methods exist for steel production: the blast furnace and the electric arc furnace. Both methods vary in their approach but converge to produce molten steel, which is subsequently cast and rolled into various shapes.
Hallmarks of Steel
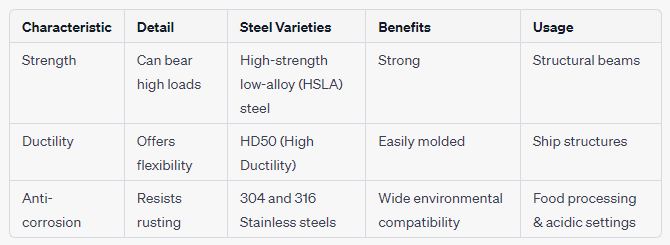
Strength: Renowned for its tensile strength.
Durability: Can last for a century or more.
Versatility: Its array of grades caters to countless applications.
Machinability: Many grades are effortlessly machined.
Weldability: Predominantly, steel is weld-friendly.
Corrosion Resistance: Enhancements through alloying.
Conductivity: Generally a poor conductor, making it ideal as a heat-resistant shield.
Recycling: Highly recyclable, with a substantial global recycle rate.
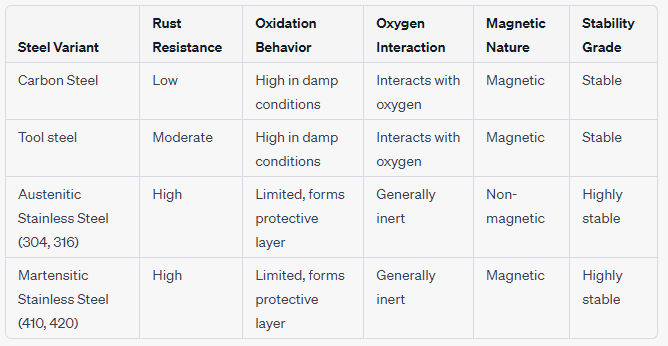
Visual Characteristics of Steel Typically, steel sports a silver-gray hue. However, its final look can vary based on the type and its exposure to environmental elements. For instance, while polished stainless steel gleams, carbon steel darkens over time due to oxidation.
A Spectrum of Steel Types
Stainless Steel: Primarily contains chromium and nickel.
Carbon Steel: Defined by its carbon content.
Alloy Steel: Features additional alloying elements.
Tool Steel: Tailored for tool manufacturing.
Weathering Steel: Designed for outdoor durability.
Electrical Steel: Enriched with silicon for electrical applications.
High-Speed Steel: Ideal for rapidly moving tools.
Key Properties of Steel Steels manifest a variety of properties. Table 1 encapsulates some of these attributes.
Table 1: Overview of Steel Characteristics & Applications
Table 2: Physical Qualities of Steel
Table 3: Chemical Aspects of Steel
Steel’s Broad Applications:
Transport: Steel's robustness is essential for creating bridges, railways, vehicle frames, and ships.
Construction: Its resilience and rigidity make it ideal for structural applications.
Manufacturing: Steel is prevalent in machinery and tooling due to its strength.
Packaging: Steel's durability and recyclability make it perfect for canning.
Medical Tools: Stainless steel is favored for its hygienic and corrosion-resistant properties.
Sports Gear: Equipment like bicycles and golf clubs value steel's durability.
Energy Generation: Its properties make steel crucial for generators, transformers, and electricity transmission.
Steel's Advantages:
Durability: Steel can endure significant stresses without deforming.
Adaptability: It can be tailored, machined, and joined with ease.
Stability: It maintains its shape and size against external forces.
Recyclability: Steel can be endlessly recycled, minimizing waste.
Safety: Non-flammable and non-toxic, steel ensures safe construction.
Steel's Drawbacks:
Corrosion: Requires protection in damp environments.
Heaviness: Its weight may limit its utility in weight-sensitive sectors.
Energy Intensive: Steel production can be energy-consuming.
Heat Conduction: Insulating steel structures can be challenging.
Expense: Specialized steel types can be costly.
Why Opt for Steel? It's versatile, robust, and available in various grades. Depending on the type, steel can also resist corrosion and maintain its dimensions effectively.
Queries about Steel:
Is Steel a Metal? Yes, primarily composed of iron, steel is a metallic alloy.
Does Steel Rust? Yes, especially in the presence of moisture, but stainless steels resist rusting.
Is Steel Tougher Than Iron? Yes, thanks to its carbon content.
Are Any Materials Harder than Steel? Yes, materials like diamond and tungsten surpass steel in hardness.
How Do Steel and Metal Differ? While all steels are metals, not every metal is steel.
Steel vs. Aluminum: Steel is a carbon and iron alloy, while aluminum is a distinct metallic element. Steel is more robust but heavier, whereas aluminum is lighter with lower strength.
Optical emission spectrometric
Optical Emission Spectroscopy (OES) is a widely used analytical technique in metallurgy that enables accurate and time-efficient determination of the elemental composition of various metals and alloys. Often referred to as a "spark test," OES employs an electrical discharge to create a distinct chemical signature by vaporizing a small amount of material. This article explores the applications of OES in metallurgy, particularly its role in quality control processes in steel making and aluminum metallurgy.
I. Understanding Optical Emission Spectroscopy (OES)
Definition and principles of OES
Sparking process and its role in generating a chemical signature
II. Applications of OES in Metallurgy
A. Quality Control in Steel Making
Rapid elemental analysis
Ensuring alloy composition compliance
Monitoring impurities and trace elements
Identification of non-metallic inclusions
B. Aluminum Metallurgy Processes
Elemental composition analysis of aluminum alloys
Detection of impurities and trace elements
Verification of alloy grade specifications
Control of alloying elements for desired properties
III. Advantages of OES in Metallurgy
Time efficiency in elemental analysis
High accuracy and precision
Non-destructive testing capability
Wide range of detectable elements
IV. OES Instrumentation and Methodology
Overview of OES equipment and spectrometers
Sample preparation techniques
Measurement parameters and calibration procedures
V. Future Developments and Challenges
Advancements in OES technology
Integration with automation and data analysis tools
Overcoming limitations for complex alloy systems
Optical Emission Spectroscopy (OES) plays a vital role in metallurgy, offering a rapid and accurate method for elemental analysis in various metal samples. Its application in quality control processes for steel making and aluminum metallurgy ensures compliance with alloy composition requirements, monitors impurities, and verifies grade specifications. As OES technology continues to advance, it holds great potential for further enhancing the efficiency and precision of elemental analysis in the field of metallurgy.
X-radial fluorescence spectrometric
XRF Testing on Metal: Ensuring Material Integrity through Positive Material Identification (PMI)
Introduction
Positive Material Identification (PMI) is a crucial aspect of manufacturing, petrochemical production, and consumer product industries. It is essential to use the correct metal or alloy in the appropriate applications while ensuring the absence of composition aberrations like heavy metal contamination. X-ray Fluorescence (XRF) testing has emerged as a highly effective and user-friendly method for component PMI, providing rapid confirmation of the right metal or alloy selection.
I. Advantages of Handheld XRF
Portability and ease of use
Minimal sample preparation requirements
Swift results with rapid alloy identification capabilities
II. Comprehensive Material Composition Analysis
XRF's ability to quantify over 90% of periodic table elements
Representative limits of detection for common alloying elements (Figure 1)
Positive grade matching for various alloys including aluminum, stainless steel, copper alloys, titanium alloys, and more
Limitations in directly measuring light elements and alternative analytical methods
III. Sample Condition Considerations
Surface measurement nature of XRF
Depth of analysis based on the type of metal
Impact of surface contamination on accurate analysis
Importance of sample cleanliness and removal of external factors
Overcoming challenges with complex sample geometries
IV. Sample Surface Temperature Effects
Unaffected X-ray physics by sample temperature variations
Reliable performance of Vanta XRF analyzers in various environmental conditions
Temperature limitations and the availability of alternative face plates for hot testing
Conclusion
X-ray Fluorescence (XRF) testing serves as a powerful tool for Positive Material Identification (PMI) in the metal industry. With its extensive analytical capabilities and user-friendly nature, XRF enables swift and confident PMI, preventing production losses, injuries, or even loss of life resulting from the use of incorrect component materials. By employing XRF, industries can ensure the integrity of their materials, bolstering quality control and overall safety standards.
Carbon sulphur analyser
Introduction
In the realm of steel production, the measurement of carbon and sulfur concentrations plays a critical role in ensuring optimal material properties. As steel undergoes the transformation process from raw iron with high carbon content to stainless steel with only trace amounts of carbon, understanding the precise levels of carbon becomes crucial. Carbon content not only influences the texture of the material but also determines key characteristics such as hardness, elasticity, and magnetism. To analyze carbon and sulfur concentrations swiftly and accurately, various methods have been developed. This article focuses on the carbon-sulfur analysis method, shedding light on the advanced combustion analyzers, such as ELTRA's ELEMENTRAC CS-i, that enable quick and efficient measurements. By employing these modern techniques, industries can effectively monitor carbon and sulfur levels, ensuring the desired material quality and performance.
Measuring Carbon and Sulfur Concentrations
In the quest to determine carbon and sulfur concentrations in steel and iron-based products, several methods have emerged. Multi-element techniques like spark Optical Emission Spectroscopy (OES) and Glow Discharge Optical Emission Spectroscopy (GDOES) have been widely used. These techniques involve removing a small portion of the sample's surface and exciting the atoms within through optical emission. By measuring the emitted wavelengths specific to each element, carbon and sulfur, along with other elements like manganese and chromium, can be simultaneously analyzed. However, these multi-element techniques have limitations when it comes to certain sample shapes or the presence of elemental impurities of carbon and sulfur.
Alternatively, spectrometric methods like Inductively Coupled Plasma Optical Emission Spectroscopy (ICP OES) offer more flexibility in terms of sample shapes but require a dissolved sample. Nevertheless, when analyzing low carbon and sulfur concentrations, such as those found in stainless steel, the presence of blank values from the used acids and solvents must be considered.
To overcome these limitations, carbon-sulfur analyzers, also known as combustion analyzers, have emerged as an efficient solution. These analyzers utilize a different measurement principle, where the sample is melted using an induction furnace with a high flow of oxygen. This process combusts the bound carbon and sulfur into carbon dioxide and sulfur dioxide, which can be detected using electronic detectors such as infrared cells or thermal conductivity cells. Unlike traditional techniques that could take up to 90 minutes to analyze one element, modern combustion analyzers provide results within a matter of seconds.
Modern Combustion Analyzers
ELEMENTRAC CS-i In contrast to the time-consuming methods of the past, modern combustion analyzers like the ELEMENTRAC CS-i offer significant advantages, providing rapid and reliable measurements of carbon and sulfur concentrations. The ELEMENTRAC CS-i features a power-controllable induction furnace with intelligent lance management, a heated dust trap, and an integrated catalyst. Equipped with up to four infrared cells, this analyzer ensures a wide measuring range, allowing for precise measurements ranging from several parts per million (ppm) to percentage levels. The ELEMENTRAC CS-i complies with relevant international standards and literature, making it a reliable tool for both academic and non-academic staff.
The analysis process with the ELEMENTRAC CS-i involves a few straightforward steps. After weighing the sample in a ceramic crucible and logging it into the software, an accelerator is added, and the measuring process begins. The analyzer's software and hardware handle the subsequent steps, minimizing the need for user intervention. This user-friendly approach simplifies the measurement process while maintaining accuracy and precision.
Conclusion
The advancement of modern combustion analyzers, exemplified by the ELEMENTRAC CS-i, has revolutionized the measurement of carbon and sulfur concentrations in metals. By enabling rapid and reliable analysis within
Nitrogen oxygen analyser
LECO analysis is a dependable technique employed to measure the concentration of elements in metallic samples, encompassing Carbon, Hydrogen, Nitrogen, Oxygen, and Sulfur. The analysis involves utilizing infrared absorption and thermal conductivity to evaluate the combustion gases within the sample, allowing for the determination of the presence and concentration of these elements. LECO analysis achieves this by converting the elements in the sample into their oxidized state using two distinct methods: the gas fusion method (for Hydrogen, Nitrogen, and Oxygen) and the combustion method (for Carbon and Sulfur).